- Record: found
- Abstract: found
- Article: found
In Vivo Injection of Anti-LGI1 Antibodies into the Rodent M1 Cortex and Hippocampus Is Ineffective in Inducing Seizures
Read this article at
Abstract
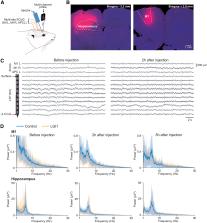
Autoimmune encephalitis (AIE) associated with antibodies directed against the leucine-rich glioma inactivated 1 (LGI1) protein is the second most common AIE and is responsible for deleterious neocortical and limbic epileptic seizures. Previous studies demonstrated a pathogenic role of anti-LGI1 antibodies via alterations in the expression and function of Kv1 channels and AMPA receptors. However, the causal link between antibodies and epileptic seizures has never been demonstrated. Here, we attempted to determine the role of human anti-LGI1 autoantibodies in the genesis of seizures by analyzing the impact of their intracerebral injection in rodents. Acute and chronic injections were performed in rats and mice in the hippocampus and primary motor cortex, the two main brain regions affected by the disease. Acute infusion of CSF or serum IgG of anti-LGI1 AIE patients did not lead to the emergence of epileptic activities, as assessed by multisite electrophysiological recordings over a 10 h period after injection. A chronic 14 d injection, coupled with continuous video-EEG monitoring, was not more effective. Overall, these results demonstrate that acute and chronic injections of CSF or purified IgG from LGI1 patients are not able to generate epileptic activity by themselves in the different animal models tested.
Related collections
Most cited references39

- Record: found
- Abstract: found
- Article: found
FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data
- Record: found
- Abstract: found
- Article: not found
Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice.
- Record: found
- Abstract: found
- Article: not found