- Record: found
- Abstract: found
- Article: not found
Depolarization-induced Calcium Responses in Sympathetic Neurons: Relative Contributions from Ca 2+ Entry, Extrusion, ER/Mitochondrial Ca 2+ Uptake and Release, and Ca 2+ Buffering
Read this article at
Abstract
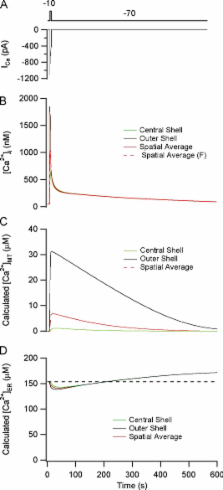
Many models have been developed to account for stimulus-evoked [Ca 2+] responses, but few address how responses elicited in specific cell types are defined by the Ca 2+ transport and buffering systems that operate in the same cells. In this study, we extend previous modeling studies by linking the time course of stimulus-evoked [Ca 2+] responses to the underlying Ca 2+ transport and buffering systems. Depolarization-evoked [Ca 2+] i responses were studied in sympathetic neurons under voltage clamp, asking how response kinetics are defined by the Ca 2+ handling systems expressed in these cells. We investigated five cases of increasing complexity, comparing observed and calculated responses deduced from measured Ca 2+ handling properties. In Case 1, [Ca 2+] i responses were elicited by small Ca 2+ currents while Ca 2+ transport by internal stores was inhibited, leaving plasma membrane Ca 2+ extrusion intact. In Case 2, responses to the same stimuli were measured while mitochondrial Ca 2+ uptake was active. In Case 3, responses were elicited as in Case 2 but with larger Ca 2+ currents that produce larger and faster [Ca 2+] i elevations. Case 4 included the mitochondrial Na/Ca exchanger. Finally, Case 5 included ER Ca 2+ uptake and release pathways. We found that [Ca 2+] i responses elicited by weak stimuli (Cases 1 and 2) could be quantitatively reconstructed using a spatially uniform model incorporating the measured properties of Ca 2+ entry, removal, and buffering. Responses to strong depolarization (Case 3) could not be described by this model, but were consistent with a diffusion model incorporating the same Ca 2+ transport and buffering descriptions, as long as endogenous buffers have low mobility, leading to steep radial [Ca 2+] i gradients and spatially nonuniform Ca 2+ loading by mitochondria. When extended to include mitochondrial Ca 2+ release (Case 4) and ER Ca 2+ transport (Case 5), the diffusion model could also account for previous measurements of stimulus-evoked changes in total mitochondrial and ER Ca concentration.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Generation, control, and processing of cellular calcium signals.
- Record: found
- Abstract: found
- Article: not found
Calcium gradients and buffers in bovine chromaffin cells.
- Record: found
- Abstract: found
- Article: not found
