- Record: found
- Abstract: found
- Article: found
DHPR activation underlies SR Ca 2+ release induced by osmotic stress in isolated rat skeletal muscle fibers
Read this article at
Abstract
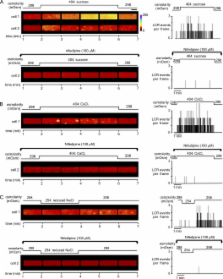
Changes in skeletal muscle volume induce localized sarcoplasmic reticulum (SR) Ca 2+ release (LCR) events, which are sustained for many minutes, suggesting a possible signaling role in plasticity or pathology. However, the mechanism by which cell volume influences SR Ca 2+ release is uncertain. In the present study, rat flexor digitorum brevis fibers were superfused with isoosmotic Tyrode's solution before exposure to either hyperosmotic (404 mOsm) or hypoosmotic (254 mOsm) solutions, and the effects on cell volume, membrane potential (E m), and intracellular Ca 2+ ([Ca 2+] i) were determined. To allow comparison with previous studies, solutions were made hyperosmotic by the addition of sugars or divalent cations, or they were made hypoosmotic by reducing [NaCl] o. All hyperosmotic solutions induced a sustained decrease in cell volume, which was accompanied by membrane depolarization (by 14–18 mV; n = 40) and SR Ca 2+ release. However, sugar solutions caused a global increase in [Ca 2+] i, whereas solutions made hyperosmotic by the addition of divalent cations only induced LCR. Decreasing osmolarity induced an increase in cell volume and a negative shift in E m (by 15.04 ± 1.85 mV; n = 8), whereas [Ca 2+] i was unaffected. However, on return to the isoosmotic solution, restoration of cell volume and E m was associated with LCR. Both global and localized SR Ca 2+ release were abolished by the dihydropyridine receptor inhibitor nifedipine by sustained depolarization of the sarcolemmal or by the addition of the ryanodine receptor 1 inhibitor tetracaine. Inhibitors of the Na-K-2Cl (NKCC) cotransporter markedly inhibited the depolarization associated with hyperosmotic shrinkage and the associated SR Ca 2+ release. These findings suggest (1) that the depolarization that accompanies a decrease in cell volume is the primary event leading to SR Ca 2+ release, and (2) that volume-dependent regulation of the NKCC cotransporter contributes to the observed changes in E m. The differing effects of the osmotic agents can be explained by the screening of fixed charges by divalent ions.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise.
- Record: found
- Abstract: found
- Article: not found
Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension.
- Record: found
- Abstract: found
- Article: not found