- Record: found
- Abstract: found
- Article: found
Pharmacokinetics, Tissue Distribution, and Excretion Characteristics of a Radix Polygoni Multiflori Extract in Rats
Read this article at
Abstract
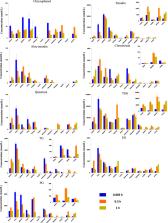
Although progress has been achieved in the pharmacological activity and toxicity of Radix Polygoni Multiflori (RPM), the chemical basis of its toxicity is still unclear. Here, we performed a multicompound pharmacokinetic analysis and investigated the tissue distribution and excretion characteristics of RPM components after oral administration in rats. The findings demonstrated that the active ingredients of the RPM extract were quickly absorbed after oral administration, with high exposure levels of emodin, 2,3,5,4′-teterahydroxystilbene-2-O-β-D-glucoside (TSG), citreorosein, torachrysone-8-O-glucoside (TG), emodin-8-O-β-D-glucoside (EG), and physcion-8-O-β-D-glucoside (PG). The tissue distributions of emodin, TSG, TG, EG, and PG were high in the liver and kidney. These components were the key contributors to the effectiveness and toxicity of RPM on the liver and kidney. Most of the active ingredients were mainly excreted through feces and bile, while a few were converted into other products in the body and excreted through urine and feces.
Related collections
Most cited references75
- Record: found
- Abstract: found
- Article: not found
Dose translation from animal to human studies revisited.
- Record: found
- Abstract: not found
- Book: not found
Pharmacopoeia of the People’s Republic of China
- Record: found
- Abstract: found
- Article: not found