- Record: found
- Abstract: found
- Article: found
Site Specific Cleavage Mediated by MMPs Regulates Function of Agrin
Read this article at
Abstract
Background
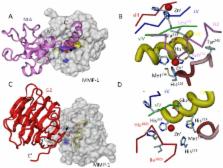
Agrin is the key inducer of postsynaptic differentiations at the neuromuscular junction. The multidomain heparan sulfate proteoglycan is mediating via its N-terminal segment the interaction with laminin, whereas the C-terminal portion is responsible for Dystroglycan binding and clustering of the Acetylcholine receptor. Matrix metalloproteinases (MMP) are known to play essential roles in matrix remodeling, degradation and regulation of extracellular signaling networks.
Principal Findings
Site-specific processing of Agrin provides key insight into regulatory effects of Matrix metalloproteinases (MMPs). Here, we present a detailed study of agrin processing by different MMPs together with a molecular understanding of binding and cleavage at both terminal fragments. The data suggest for a regulatory effect of MMP cleavage at particularly important functional sites of agrin. Cleave of agrin abolishes the agrin-laminin complex formation and the Acetylcholine receptor clustering at the neuromuscular junction.
Related collections
Most cited references38
- Record: found
- Abstract: not found
- Article: not found
Matrix metalloproteinases.
- Record: found
- Abstract: found
- Article: not found
Lrp4 is a receptor for Agrin and forms a complex with MuSK.
- Record: found
- Abstract: found
- Article: not found
