- Record: found
- Abstract: found
- Article: found
The Neuroendocrine Protein 7B2 Suppresses the Aggregation of Neurodegenerative Disease-related Proteins*
Read this article at
Abstract
Background: The neuroendocrine protein 7B2 blocks the aggregation of certain secreted proteins.
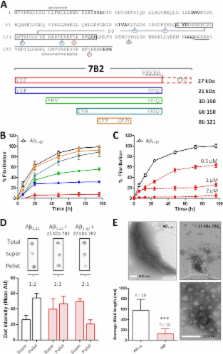
Results: 7B2 co-localizes with protein aggregates in Parkinson and Alzheimer disease brains; blocks the fibrillation of Aβ 1–40, Aβ 1–42, and α-synuclein; and blocks Aβ 1–42-induced Neuro-2A cell death.
Conclusion: 7B2 inhibits the cytotoxicity of Aβ 1–42 by modulation of oligomer formation.
Significance: 7B2 is a novel anti-aggregation secretory chaperone associated with neurodegenerative disease.
Abstract
Neurodegenerative diseases such as Alzheimer (AD) and Parkinson (PD) are characterized by abnormal aggregation of misfolded β-sheet-rich proteins, including amyloid-β (Aβ)-derived peptides and tau in AD and α-synuclein in PD. Correct folding and assembly of these proteins are controlled by ubiquitously expressed molecular chaperones; however, our understanding of neuron-specific chaperones and their involvement in the pathogenesis of neurodegenerative diseases is limited. We here describe novel chaperone-like functions for the secretory protein 7B2, which is widely expressed in neuronal and endocrine tissues. In in vitro experiments, 7B2 efficiently prevented fibrillation and formation of Aβ 1–42, Aβ 1–40, and α-synuclein aggregates at a molar ratio of 1:10. In cell culture experiments, inclusion of recombinant 7B2, either in the medium of Neuro-2A cells or intracellularly via adenoviral 7B2 overexpression, blocked the neurocytotoxic effect of Aβ 1–42 and significantly increased cell viability. Conversely, knockdown of 7B2 by RNAi increased Aβ 1–42-induced cytotoxicity. In the brains of APP/PSEN1 mice, a model of AD amyloidosis, immunoreactive 7B2 co-localized with aggregation-prone proteins and their respective aggregates. Furthermore, in the hippocampus and substantia nigra of human AD- and PD-affected brains, 7B2 was highly co-localized with Aβ plaques and α-synuclein deposits, strongly suggesting physiological association. Our data provide insight into novel functions of 7B2 and establish this neural protein as an anti-aggregation chaperone associated with neurodegenerative disease.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Modulation of neurodegeneration by molecular chaperones.
- Record: found
- Abstract: found
- Article: not found
Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease.
- Record: found
- Abstract: found
- Article: not found