- Record: found
- Abstract: found
- Article: found
A novel mechanism of “metal gel-shift” by histidine-rich Ni 2+-binding Hpn protein from Helicobacter pylori strain SS1
Read this article at
Abstract
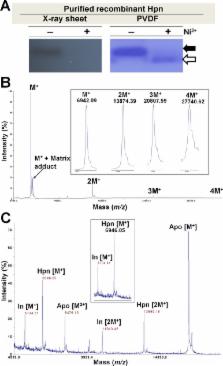
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) is a universally used method for determining approximate molecular weight (MW) in protein research. Migration of protein that does not correlate with formula MW, termed “gel shifting” appears to be common for histidine-rich proteins but not yet studied in detail. We investigated “gel shifting” in Ni 2+-binding histidine-rich Hpn protein cloned from Helicobacter pylori strain SS1. Our data demonstrate two important factors determining “gel shifting” of Hpn, polyacrylamide-gel concentration and metal binding. Higher polyacrylamide-gel concentrations resulted in faster Hpn migration. Irrespective of polyacrylamide-gel concentration, preserved Hpn-Ni 2+ complex migrated faster (3–4 kDa) than apo-Hpn, phenomenon termed “metal gel-shift” demonstrating an intimate link between Ni 2+ binding and “gel shifting”. To examine this discrepancy, eluted samples from corresponding spots on SDS-gel were analyzed by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF-MS). The MW of all samples was the same (6945.66±0.34 Da) and identical to formula MW with or without added mass of Ni 2+. MALDI-TOF-MS of Ni 2+-treated Hpn revealed that monomer bound up to six Ni 2+ ions non-cooperatively, and equilibrium between protein-metal species was reliant on Ni 2+ availability. This corroborates with gradually increased heterogeneity of apo-Hpn band followed by compact "metal-gel shift" band on SDS-PAGE. In view of presented data metal-binding and “metal-gel shift” models are discussed.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Detergent binding explains anomalous SDS-PAGE migration of membrane proteins.
- Record: found
- Abstract: found
- Article: not found
Measurements of volatile organic compounds in the earth's atmosphere using proton-transfer-reaction mass spectrometry.
- Record: found
- Abstract: found
- Article: not found