- Record: found
- Abstract: found
- Article: found
Preclinical imaging of the co-stimulatory molecules CD80 and CD86 with indium-111-labeled belatacept in atherosclerosis
Read this article at
Abstract
Background
The inflammatory nature of atherosclerosis provides a broad range of potential molecular targets for atherosclerosis imaging. Growing interest is focused on targets related to plaque vulnerability such as the co-stimulatory molecules CD80 and CD86. We investigated in this preclinical proof-of-concept study the applicability of the CD80/CD86-binding fusion protein belatacept as a probe for atherosclerosis imaging.
Methods
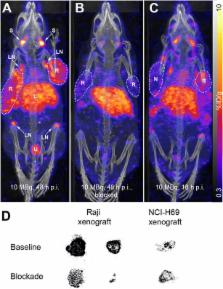
Belatacept was labeled with indium-111, and the binding affinity was determined with CD80/CD86-positive Raji cells. In vivo distribution was investigated in Raji xenograft-bearing mice in single-photon emission computed tomography (SPECT)/CT scans, biodistribution, and ex vivo autoradiography studies. Ex vivo SPECT/CT experiments were performed with aortas and carotids of ApoE KO mice. Accumulation in human carotid atherosclerotic plaques was investigated by in vitro autoradiography.
Results
111In-DOTA-belatacept was obtained in >70 % yield, >99 % radiochemical purity, and ~40 GBq/μmol specific activity. The labeled belatacept bound with high affinity to Raji cells. In vivo, 111In-DOTA-belatacept accumulated specifically in Raji xenografts, lymph nodes, and salivary glands. Ex vivo SPECT experiments revealed displaceable accumulation in atherosclerotic plaques of ApoE KO mice fed an atherosclerosis-promoting diet. In human plaques, binding correlated with the infiltration by immune cells and the presence of a large lipid and necrotic core.
Conclusions
111In-DOTA-belatacept accumulates in CD80/CD86-positive tissues in vivo and in vitro rendering it a research tool for the assessment of inflammatory activity in atherosclerosis and possibly other diseases. The tracer is suitable for preclinical imaging of co-stimulatory molecules of both human and murine origin. Radiolabeled belatacept could serve as a benchmark for future CD80/CD86-specific imaging agents.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties.
- Record: found
- Abstract: found
- Article: not found
The B7 family of immune-regulatory ligands
- Record: found
- Abstract: found
- Article: not found