- Record: found
- Abstract: found
- Article: found
Control of Vascular Smooth Muscle Cell Growth by Connexin 43
Read this article at
Abstract
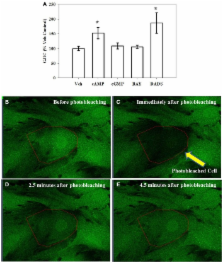
Connexin 43 (Cx43), the principal gap junction protein in vascular smooth muscle cells (VSMCs), regulates movement of ions and other signaling molecules through gap junction intercellular communication (GJIC) and plays important roles in maintaining normal vessel function; however, many of the signaling mechanisms controlling Cx43 in VSMCs are not clearly described. The goal of this study was to investigate mechanisms of Cx43 regulation with respect to VSMC proliferation. Treatment of rat primary VSMCs with the cAMP analog 8Br-cAMP, the soluble guanylate cyclase (sGC) stimulator BAY 41-2272 (BAY), or the Cx inducer diallyl disulfide (DADS) significantly reduced proliferation after 72 h compared with vehicle controls. Bromodeoxyuridine uptake revealed reduction ( p < 0.05) in DNA synthesis after 6 h and flow cytometry showed reduced (40%) S-phase cell numbers after 16 h in DADS-treated cells compared with vehicle controls. Cx43 expression significantly increased after 270 min treatment with 8Br-cAMP, 8Br-cGMP, BAY or DADS. Inhibition of PKA, PKG or PKC reversed 8Br-cAMP-stimulated increases in Cx43 expression, whereas only PKG or PKC inhibition reversed 8Br-cGMP- and BAY-stimulated increases in total Cx43. Interestingly, stimulation of Cx43 expression by DADS was not dependent on PKA, PKG or PKC. Using fluorescence recovery after photobleaching, only 8Br-cAMP or DADS increased GJIC with 8Br-cAMP mediated by PKC and DADS mediated by PKG. Further, DADS significantly increased phosphorylation at MAPK-sensitive Serine (Ser)255 and Ser279, the cell cycle regulatory kinase-sensitive Ser262 and PKC-sensitive Ser368 after 30 min while 8Br-cAMP significantly increased phosphorylation only at Ser279 compared with controls. This study demonstrates that 8Br-cAMP- and DADS-enhanced GJIC rather than Cx43 expression and/or phosphorylation plays important roles in the regulation of VSMC proliferation and provides new insights into the growth-regulatory capacities of Cx43 in VSM.
Related collections
Most cited references76
- Record: found
- Abstract: not found
- Article: not found
The gap junction communication channel.
- Record: found
- Abstract: found
- Article: not found
Gap junctions: structure and function (Review).
- Record: found
- Abstract: found
- Article: not found