- Record: found
- Abstract: found
- Article: found
Nanoparticle-Mediated Delivery of Emodin via Colonic Irrigation Attenuates Renal Injury in 5/6 Nephrectomized Rats
Read this article at
Abstract
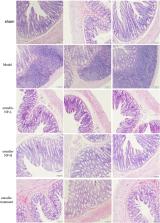
Our previous study showed that emodin enema modulates gut microbiota and delays CKD progression. However, the poor solubility, limited colonic irrigation retention time, and inadequate colon adhesion of emodin hinder its clinical application. Based on the deficiencies of emodin, we prepared monomethoxy-poly (ethylene glycol)-poly (lactic acid)-chitosan-2-mercaptobenzimidazole nanoparticles with incorporated emodin (emodin-NP) and studied their efficacy in delaying CKD progression. 5/6 nephrectomized Male Sprague Dawley rats were administered via colonic irrigation with emodin-NP every two days for eight weeks. We found that treatment with emodin-NP improved the kidney function of the rats and limited the expansion of tubulointerstitial fibrosis. Treatment with emodin-NP once every two days is comparable to emodin treatment once a day. Furthermore, emodin-NP via colonic irrigation remarkably reduced IL-1β, IL-6, and LPS levels in serum, improved intestinal barrier functions, and downregulated the key proteins (TLR4, MyD88, and NF-κB) expression in intestinal TLR4 signaling pathway. 16S rDNA analyses showed that emodin-NP can regulate microbiota disturbance in CKD. Taken together, these results suggest that emodin-NP alleviates kidney dysfunction and tubulointerstitial fibrosis by mediation through the modification of gut microbiota disorders. Emodin-NP may be a new method to treat CKD.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study
- Record: found
- Abstract: not found
- Article: not found
Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids

- Record: found
- Abstract: found
- Article: found