- Record: found
- Abstract: found
- Article: found
Cardiomyopathy phenotypes in human-induced pluripotent stem cell-derived cardiomyocytes—a systematic review
Read this article at
Abstract
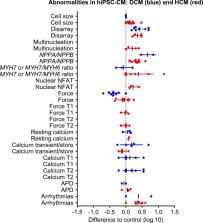
Human-induced pluripotent stem cells (hiPSC) can be differentiated to cardiomyocytes at high efficiency and are increasingly used to study cardiac disease in a human context. This review evaluated 38 studies on hypertrophic (HCM) and dilated cardiomyopathy (DCM) of different genetic causes asking to which extent published data allow the definition of an in vitro HCM/DCM hiPSC-CM phenotype. The data are put in context with the prevailing hypotheses on HCM/DCM dysfunction and pathophysiology. Relatively consistent findings in HCM not reported in DCM were larger cell size (156 ± 85%, n = 15), more nuclear localization of nuclear factor of activated T cells (NFAT; 175 ± 65%, n = 3), and higher β-myosin heavy chain gene expression levels (500 ± 547%, n = 8) than respective controls. Conversely, DCM lines showed consistently less force development than controls (47 ± 23%, n = 9), while HCM forces scattered without clear trend. Both HCM and DCM lines often showed sarcomere disorganization, higher NPPA/NPPB expression levels, and arrhythmic beating behaviour. The data have to be taken with the caveat that reporting frequencies of the various parameters (e.g. cell size, NFAT expression) differ widely between HCM and DCM lines, in which data scatter is large and that only 9/38 studies used isogenic controls. Taken together, the current data provide interesting suggestions for disease-specific phenotypes in HCM/DCM hiPSC-CM but indicate that the field is still in its early days. Systematic, quantitative comparisons and robust, high content assays are warranted to advance the field.
Related collections
Most cited references80
- Record: found
- Abstract: found
- Article: found
Common genetic variation drives molecular heterogeneity in human iPSCs
- Record: found
- Abstract: found
- Article: not found
Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy.
- Record: found
- Abstract: found
- Article: not found