- Record: found
- Abstract: found
- Article: found
Characteristics of Randomized Trials Published in Latin America and the Caribbean According to Funding Source
Read this article at
Abstract
Introduction
Few studies have assessed the nature and quality of randomized controlled trials (RCTs) in Latin America and the Caribbean (LAC).
Methods and Findings
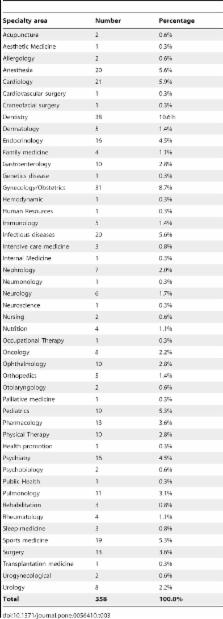
The aims of this systematic review are to evaluate the characteristics (including the risk of bias assessment) of RCT conducted in LAC according to funding source. A review of RCTs published in 2010 in which the author's affiliation was from LAC was performed in PubMed and LILACS. Two reviewers independently extracted data and assessed the risk of bias. The primary outcomes were risk of bias assessment and funding source. A total of 1,695 references were found in PubMed and LILACS databases, of which 526 were RCTs (N = 73.513 participants). English was the dominant publication language (93%) and most of the RCTs were published in non-LAC journals (84.2%). Only five of the 19 identified countries accounted for nearly 95% of all RCTs conducted in the region (Brazil 70.9%, Mexico 10.1%, Argentina 5.9%, Colombia 3.8%, and Chile 3.4%). Few RCTs covered priority areas related with Millennium Development Goals like maternal health (6.7%) or high priority infectious diseases (3.8%). Regarding children, 3.6% and 0.4% RCT evaluated nutrition and diarrhea interventions respectively but none pneumonia. As a comparison, aesthetic and sport related interventions account for 4.6% of all trials. A random sample of RCTs (n = 358) was assessed for funding source: exclusively public (33.8%); private (e.g. pharmaceutical company) (15.3%); other (e.g. mixed, NGO) (15.1%); no funding (35.8%). Overall assessments for risk of bias showed no statistically significant differences between RCTs and type of funding source. Statistically significant differences favoring private and others type of funding was found when assessing trial registration and conflict of interest reporting.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
A checklist for health research priority setting: nine common themes of good practice
- Record: found
- Abstract: found
- Article: not found
Productivity costs of cancer mortality in the United States: 2000-2020.
- Record: found
- Abstract: found
- Article: not found