- Record: found
- Abstract: found
- Article: found
Generation of induced pluripotent stem cells from renal tubular cells of a patient with Alport syndrome
Read this article at
Abstract
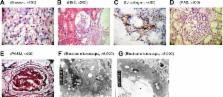
Alport syndrome (AS) is a hereditary disease that leads to kidney failure and is caused by mutations in the COL4A3, COL4A4, and COL4A5 genes that lead to the absence of collagen α3α4α5 (IV) networks in the mature kidney glomerular basement membrane. Approximately 80% of AS is X-linked because of mutations in COL4A5, the gene encoding the alpha 5 chain of type IV collagen. To investigate the pathogenesis of AS at the genetic level, we generated induced pluripotent stem cells (iPSCs) from renal tubular cells of a patient with AS. The successful iPSC generation laid the foundation to master the repair of the COL4A5 gene and to evaluate the differentiation of iPSC into Sertoli cells and the accompanying epigenetic changes at each stage. The generation of iPSCs from AS patients not only confirms that iPSCs could be generated from renal tubular cells, but also provides a novel type of genetic therapy for AS patients. In this study, we generated iPSCs from renal tubular cells via ectopic expression of four transcription factors ( Oct4, Sox2, c-myc, and Klf4). According to the human embryonic stem cell (hESC) charter, iPSC formation was confirmed by comparatively analyzing hESC markers via colony morphology, immunohistochemistry, qRT-PCR, flow cytometry, gene expression profiling of the three germ layers, and karyotyping. Our results demonstrated that iPSCs were similar to hESCs with regard to morphology, proliferation, hESC-specific surface marker expression, and differentiation into the cell types of the three germ layers. The efficient generation of iPSCs from the renal tubular cells of an AS patient would provide a novel model to investigate the mechanisms underlying AS and to develop new treatments for AS.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
Generation of induced pluripotent stem cells from urine.
- Record: found
- Abstract: found
- Article: not found
LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson's disease patients: reversal by gene correction.
- Record: found
- Abstract: found
- Article: found