- Record: found
- Abstract: found
- Article: not found
Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells
Abstract
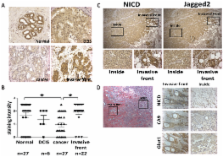
Notch signaling is often and aberrantly activated by hypoxia during tumor progression; however, the exact pathological role of hypoxia-induced Notch signaling in tumor metastasis is as yet poorly understood. In this study, we aimed to define the mechanism of Notch ligand activation by hypoxia in both primary tumor and bone stromal cells in the metastatic niche and to clarify their roles in tumor progression. We have analyzed the expression profiles of various Notch liagnds in 779 breast cancer patients in GEO database and found that the expression of Jagged2 among all five ligands is most significantly correlated with the overall- and metastasis-free survival of breast cancer patients. The results of our immunohistochemical (IHC) analysis for Jagged2 in 61 clinical samples also revealed that both Jagged2 and Notch signaling were strongly up-regulated at the hypoxic invasive front. Activation of Jagged2 by hypoxia in tumor cells induced EMT and also promoted cell survival in vitro. Notably, a γ-secretase inhibitor significantly blocked Notch-mediated invasion and survival under hypoxia by promoting expression of E-cadherin and inhibiting Akt phosphorylation. Importantly, Jagged2 was also found to be up-regulated in bone marrow stroma under hypoxia and promoted the growth of cancer stem-like cells by activating their Notch signaling. Therefore, hypoxia-induced Jagged2 activation in both tumor invasive front and normal bone stroma plays a critical role in tumor progression and metastasis, and Jagged2 is considered to be a valuable prognostic marker and may serve as a novel therapeutic target for metastatic breast cancer.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases.
- Record: found
- Abstract: found
- Article: not found
Hypoxia signalling in cancer and approaches to enforce tumour regression.
- Record: found
- Abstract: found
- Article: not found

