- Record: found
- Abstract: found
- Article: not found
Optogenetic stimulation of a hippocampal engram activates fear memory recall
Read this article at
Abstract
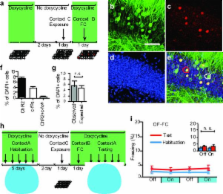
A specific memory is thought to be encoded by a sparse population of neurons 1, 2 . These neurons can be tagged during learning for subsequent identification 3 and manipulation 4, 5, 6 . Moreover, their ablation or inactivation results in reduced memory expression, suggesting their necessity in mnemonic processes. However, a critical question of sufficiency remains: can one elicit the behavioral output of a specific memory by directly activating a population of neurons that was active during learning? Here we show that optogenetic reactivation of hippocampal neurons activated during fear conditioning is sufficient to induce freezing behavior. We labeled a population of hippocampal dentate gyrus neurons activated during fear learning with channelrhodopsin-2 (ChR2) 7, 8 and later optically reactivated these neurons in a different context. The mice showed increased freezing only upon light stimulation, indicating light-induced fear memory recall. This freezing was not detected in non-fear conditioned mice expressing ChR2 in a similar proportion of cells, nor in fear conditioned mice with cells labeled by EYFP instead of ChR2. Finally, activation of cells labeled in a context not associated with fear did not evoke freezing in mice that were previously fear conditioned in a different context, suggesting that light-induced fear memory recall is context-specific. Together, our findings indicate that activating a sparse but specific ensemble of hippocampal neurons that contribute to a memory engram is sufficient for the recall of that memory. Moreover, our experimental approach offers a general method of mapping cellular populations bearing memory engrams.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Pattern separation in the dentate gyrus and CA3 of the hippocampus.
- Record: found
- Abstract: found
- Article: not found
Amygdala circuitry mediating reversible and bidirectional control of anxiety.
- Record: found
- Abstract: found
- Article: not found
