- Record: found
- Abstract: found
- Article: found
Adverse drug reactions to antiretroviral therapy (ARVs): incidence, type and risk factors in Nigeria
Read this article at
Abstract
Background
Data on adverse drug reactions (ADRs) related to antiretroviral (ARV) use in public health practice are few indicating the need for ART safety surveillance in clinical care.
Objectives
To evaluate the incidence, type and risk factors associated with adverse drug reactions (ADRs) among patients on antiretroviral drugs (ARV).
Methods
Patients initiated on ARVs between May 2006 and May 2009 were evaluated in a retrospective cohort analysis in three health facilities in Nigeria. Regimens prescribed include nucleoside backbone of zidovudine (AZT)/lamivudine (3TC), stavudine (d4T)/3TC, or tenofovir (TDF)/3TC in combination with either nevirapine (NVP) or efavirenz (EFV). Generalized Estimating Equation (GEE) model was used to identify risk factors associated with occurrence of ADR.
Results
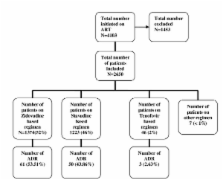
2650 patients were followed-up for 2456 person-years and reported 114 ADRs (incidence rate = 4.6/100 person-years).There were more females 1706(64%) and 73(64%) of the ADRs were reported by women. Overall, 61(54%) of ADRs were reported by patients on AZT with 54(47%) of these occurring in patients on AZT/NVP. The commonest ADRs reported were pain 25(30%) and skinrash 10(18%). Most ADRs were grade 1(39%) with only 1% being life threatening (grade 4). Adjusted GEE analysis showed that ADR was less likely to occur in patients on longer duration of ART compared to the first six months on treatment; 6-12 months AOR 0.38(95% CI:0.16-0.91) and 12-24 months AOR 0.34(95% CI:0.16-0.73) respectively. Compared to patients on TDF, ADR was less likely to occur in patients on d4T and AZT AOR 0.18(95% CI 0.05-0.64) and AOR 0.24(95% CI:0.7-0.9) respectively. Age, gender and CD4 count were not significantly associated with ADRs.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions.
- Record: found
- Abstract: found
- Article: not found