- Record: found
- Abstract: found
- Article: found
Pharmacodynamic and Pharmacokinetic Profiles of Sacubitril/Valsartan (LCZ696) in Patients with Heart Failure and Reduced Ejection Fraction
Read this article at
Summary
Aims
Concomitant renin–angiotensin–aldosterone system blockade and natriuretic peptide system enhancement may provide unique therapeutic benefits to patients with heart failure and reduced ejection fraction ( HFr EF). This study assessed the pharmacodynamics and pharmacokinetics of LCZ696 in patients with HFr EF.
Methods
This was an open‐label, noncontrolled single‐sequence study. After a 24‐h run‐in period, patients (n = 30) with HFr EF ( EF ≤ 40%; NYHA class II– IV) received LCZ696 100 mg twice daily (bid) for 7 days and 200 mg bid for 14 days, along with standard treatment for heart failure ( HF) (except angiotensin‐converting enzyme inhibitors [ ACEIs] or angiotensin receptor blockers [ ARBs]).
Results
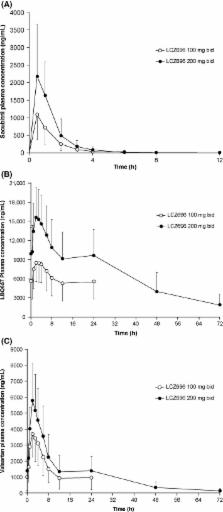
On Day 21, significant increases were observed in the plasma biomarkers indicative of neprilysin and RAAS inhibition (ratio‐to‐baseline: cyclic guanosine monophosphate [ cGMP], 1.38; renin concentration and activity, 3.50 and 2.27, respectively; all, P < 0.05). Plasma NT‐pro BNP levels significantly decreased at all the time points on Days 7 and 21; plasma aldosterone and endothelin‐1 levels significantly decreased on Day 21 (all, P < 0.05). Following administration of LCZ696, the C max of sacubitril (neprilysin inhibitor prodrug), LBQ657 (active neprilysin inhibitor), and valsartan were reached within 0.5, 2.5, and 2 h. Between 100‐ and 200‐mg doses, the C max and AUC 0–12 h for sacubitril and LBQ657 were approximately dose‐proportional while that of valsartan was less than dose‐proportional.
Conclusions
Treatment with LCZ696 for 21 days was well tolerated and resulted in plasma biomarker changes indicative of neprilysin and RAAS inhibition in patients with HF. The pharmacokinetic exposure of the LCZ696 analytes in patients with HF observed in this study is comparable to that observed in the pivotal Phase III study.
Related collections
Most cited references13
- Record: found
- Abstract: found
- Article: not found
Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions.
- Record: found
- Abstract: found
- Article: not found
Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy.
- Record: found
- Abstract: found
- Article: not found