- Record: found
- Abstract: found
- Article: found
Bench-to-bedside review: β-Adrenergic modulation in sepsis
Read this article at
Abstract
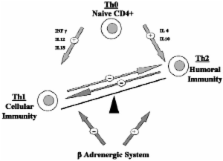
Sepsis, despite recent therapeutic progress, still carries unacceptably high mortality rates. The adrenergic system, a key modulator of organ function and cardiovascular homeostasis, could be an interesting new therapeutic target for septic shock. β-Adrenergic regulation of the immune function in sepsis is complex and is time dependent. However, β 2 activation as well as β 1 blockade seems to downregulate proinflammatory response by modulating the cytokine production profile. β 1 blockade improves cardiovascular homeostasis in septic animals, by lowering myocardial oxygen consumption without altering organ perfusion, and perhaps by restoring normal cardiovascular variability. β-Blockers could also be of interest in the systemic catabolic response to sepsis, as they oppose epinephrine which is known to promote hyperglycemia, lipid and protein catabolism. The role of β-blockers in coagulation is less clear cut. They could have a favorable role in the septic pro-coagulant state, as β 1 blockade may reduce platelet aggregation and normalize the depressed fibrinolytic status induced by adre-nergic stimulation. Therefore, β 1 blockade as well as β 2 activation improves sepsis-induced immune, cardiovascular and coagulation dysfunctions. β 2 blocking, however, seems beneficial in the metabolic field. Enough evidence has been accumulated in the literature to propose β- adrenergic modulation, β 1 blockade and β 2 activation in particular, as new promising therapeutic targets for septic dyshomeostasis, modulating favorably immune, cardiovascular, metabolic and coagulation systems.
Related collections
Most cited references92
- Record: found
- Abstract: found
- Article: not found
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.
- Record: found
- Abstract: found
- Article: not found
The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system.
- Record: found
- Abstract: found
- Article: not found