- Record: found
- Abstract: found
- Article: found
Modeling Arboviral Infection in Mice Lacking the Interferon Alpha/Beta Receptor
Abstract
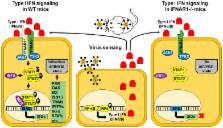
Arboviruses are arthropod-borne viruses that exhibit worldwide distribution and are a constant threat, not only for public health but also for wildlife, domestic animals, and even plants. To study disease pathogenesis and to develop efficient and safe therapies, the use of an appropriate animal model is a critical concern. Adult mice with gene knockouts of the interferon α/β (IFN-α/β) receptor (IFNAR(−/−)) have been described as a model of arbovirus infections. Studies with the natural hosts of these viruses are limited by financial and ethical issues, and in some cases, the need to have facilities with a biosafety level 3 with sufficient space to accommodate large animals. Moreover, the number of animals in the experiments must provide results with statistical significance. Recent advances in animal models in the last decade among other gaps in knowledge have contributed to the better understanding of arbovirus infections. A tremendous advantage of the IFNAR(−/−) mouse model is the availability of a wide variety of reagents that can be used to study many aspects of the immune response to the virus. Although extrapolation of findings in mice to natural hosts must be done with care due to differences in the biology between mouse and humans, experimental infections of IFNAR(−/−) mice with several studied arboviruses closely mimics hallmarks of these viruses in their natural host. Therefore, IFNAR(−/−) mice are a good model to facilitate studies on arbovirus transmission, pathogenesis, virulence, and the protective efficacy of new vaccines. In this review article, the most important arboviruses that have been studied using the IFNAR(−/−) mouse model will be reviewed.
Related collections
Most cited references131
- Record: found
- Abstract: found
- Article: not found
The nature of the principal type 1 interferon-producing cells in human blood.
- Record: found
- Abstract: found
- Article: not found