- Record: found
- Abstract: found
- Article: found
Spatial Transcriptomic Analysis of Pituitary Corticotroph Tumors Unveils Intratumor Heterogeneity
Read this article at
Abstract
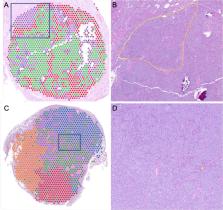
Spatial transcriptomic (ST) analysis of tumors provides a novel approach on studying gene expression along with the localization of tumor cells in their environment to uncover spatial interactions. Herein, we present ST analysis of corticotroph pituitary neuroendocrine tumors (PitNETs) from formalin-fixed, paraffin-embedded (FFPE) tissues. We report that the in situ annotation of tumor tissue can be inferred from the gene expression profiles and is in concordance with the annotation made by a pathologist. Furthermore, relative gene expression in the tumor corresponds to common protein staining used in the evaluation of PitNETs, such as reticulin and Ki-67 index. Finally, we identify intratumor heterogeneity; clusters within the same tumor may present with different secretory capacity and transcriptomic profiles, unveiling potential intratumor cell variability with possible therapeutic interest. Together, our results provide the first attempt to clarify the spatial cell profile in PitNETs.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Visualization and analysis of gene expression in tissue sections by spatial transcriptomics.
- Record: found
- Abstract: found
- Article: not found
How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression?
- Record: found
- Abstract: found
- Article: not found