- Record: found
- Abstract: found
- Article: found
Ferroportin mediates the intestinal absorption of iron from a nanoparticulate ferritin core mimetic in mice
Read this article at
Abstract
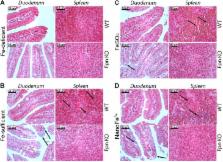
The ferritin core is composed of fine nanoparticulate Fe 3+ oxohydroxide, and we have developed a synthetic mimetic, nanoparticulate Fe 3+ polyoxohydroxide (nanoFe 3+). The aim of this study was to determine how dietary iron derived in this fashion is absorbed in the duodenum. Following a 4 wk run-in on an Fe-deficient diet, mice with intestinal-specific disruption of the Fpn-1 gene (Fpn-KO), or littermate wild-type (WT) controls, were supplemented with Fe 2+ sulfate (FeSO 4), nanoFe 3+, or no added Fe for a further 4 wk. A control group was Fe sufficient throughout. Direct intestinal absorption of nanoFe 3+ was investigated using isolated duodenal loops. Our data show that FeSO 4 and nanoFe 3+ are equally bioavailable in WT mice, and at wk 8 the mean ± sem hemoglobin increase was 18 ± 7 g/L in the FeSO 4 group and 30 ± 5 g/L in the nanoFe 3+ group. Oral iron failed to be utilized by Fpn-KO mice and was retained in enterocytes, irrespective of the iron source. In summary, although nanoFe 3+ is taken up directly by the duodenum its homeostasis is under the normal regulatory control of dietary iron absorption, namely via ferroportin-dependent efflux from enterocytes, and thus offers potential as a novel oral iron supplement.—Aslam, M. F., Frazer, D. M., Faria, N., Bruggraber, S. F. A., Wilkins, S. J., Mirciov, C., Powell, J. J., Anderson, G. J., Pereira, D. I. A. Ferroportin mediates the intestinal absorption of iron from a nanoparticulate ferritin core mimetic in mice.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter.
- Record: found
- Abstract: found
- Article: not found
A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation.
- Record: found
- Abstract: found
- Article: not found