- Record: found
- Abstract: found
- Article: found
New Insights Into the Role and Mechanism of Partial Epithelial-Mesenchymal Transition in Kidney Fibrosis
Read this article at
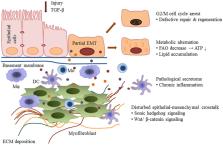
Abstract
Epithelial-mesenchymal transition (EMT) is described as the process in which injured renal tubular epithelial cells undergo a phenotype change, acquiring mesenchymal characteristics and morphing into fibroblasts. Initially, it was widely thought of as a critical mechanism of fibrogenesis underlying chronic kidney disease. However, evidence that renal tubular epithelial cells can cross the basement membrane and become fibroblasts in the renal interstitium is rare, leading to debate about the existence of EMT. Recent research has demonstrated that after injury, renal tubular epithelial cells acquire mesenchymal characteristics and the ability to produce a variety of profibrotic factors and cytokines, but remain attached to the basement membrane. On this basis, a new concept of “partial epithelial-mesenchymal transition (pEMT)” was proposed to explain the contribution of renal epithelial cells to renal fibrogenesis. In this review, we discuss the concept of pEMT and the most recent findings related to this process, including cell cycle arrest, metabolic alternation of epithelial cells, infiltration of immune cells, epigenetic regulation as well as the novel signaling pathways that mediate this disturbed epithelial-mesenchymal communication. A deeper understanding of the role and the mechanism of pEMT may help in developing novel therapies to prevent and halt fibrosis in kidney disease.
Related collections
Most cited references87
- Record: found
- Abstract: found
- Article: not found
Origin and function of myofibroblasts in kidney fibrosis.
- Record: found
- Abstract: found
- Article: not found
Mechanisms of tubulointerstitial fibrosis.
- Record: found
- Abstract: found
- Article: not found