- Record: found
- Abstract: found
- Article: found
Chronic jet lag alters gut microbiome and mycobiome and promotes the progression of MAFLD in HFHFD-fed mice
Read this article at
Abstract
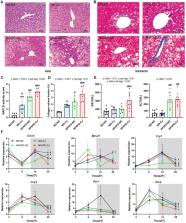
Metabolic dysfunction-associated fatty liver disease (MAFLD) is the most common chronic liver disease worldwide. Circadian disruptors, such as chronic jet lag (CJ), may be new risk factors for MAFLD development. However, the roles of CJ on MAFLD are insufficiently understood, with mechanisms remaining elusive. Studies suggest a link between gut microbiome dysbiosis and MAFLD, but most of the studies are mainly focused on gut bacteria, ignoring other components of gut microbes, such as gut fungi (mycobiome), and few studies have addressed the rhythm of the gut fungi. This study explored the effects of CJ on MAFLD and its related microbiotic and mycobiotic mechanisms in mice fed a high fat and high fructose diet (HFHFD). Forty-eight C57BL6J male mice were divided into four groups: mice on a normal diet exposed to a normal circadian cycle (ND-NC), mice on a normal diet subjected to CJ (ND-CJ), mice on a HFHFD exposed to a normal circadian cycle (HFHFD-NC), and mice on a HFHFD subjected to CJ (HFHFD-CJ). After 16 weeks, the composition and rhythm of microbiota and mycobiome in colon contents were compared among groups. The results showed that CJ exacerbated hepatic steatohepatitis in the HFHFD-fed mice. Compared with HFHFD-NC mice, HFHFD-CJ mice had increases in Aspergillus, Blumeria and lower abundances of Akkermansia, Lactococcus, Prevotella, Clostridium, Bifidobacterium, Wickerhamomyces, and Saccharomycopsis genera. The fungi-bacterial interaction network became more complex after HFHFD and/or CJ interventions. The study revealed that CJ altered the composition and structure of the gut bacteria and fungi, disrupted the rhythmic oscillation of the gut microbiota and mycobiome, affected interactions among the gut microbiome, and promoted the progression of MAFLD in HFHFD mice.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
Design and validation of a histological scoring system for nonalcoholic fatty liver disease.
- Record: found
- Abstract: found
- Article: not found
Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention
- Record: found
- Abstract: found
- Article: not found