- Record: found
- Abstract: found
- Article: found
Metabolic signatures across the full spectrum of non-alcoholic fatty liver disease
Read this article at
Abstract
Background & Aims
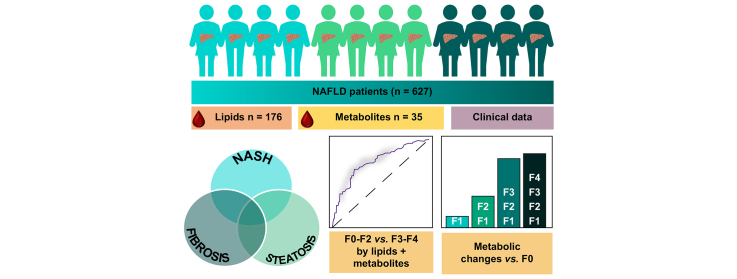
Non-alcoholic fatty liver disease (NAFLD) is a progressive liver disease with potentially severe complications including cirrhosis and hepatocellular carcinoma. Previously, we have identified circulating lipid signatures associating with liver fat content and non-alcoholic steatohepatitis (NASH). Here, we develop a metabolomic map across the NAFLD spectrum, defining interconnected metabolic signatures of steatosis (non-alcoholic fatty liver, NASH, and fibrosis).
Methods
We performed mass spectrometry analysis of molecular lipids and polar metabolites in serum samples from the European NAFLD Registry patients (n = 627), representing the full spectrum of NAFLD. Using various univariate, multivariate, and machine learning statistical approaches, we interrogated metabolites across 3 clinical perspectives: steatosis, NASH, and fibrosis.
Results
Following generation of the NAFLD metabolic network, we identify 15 metabolites unique to steatosis, 18 to NASH, and 15 to fibrosis, with 27 common to all. We identified that progression from F2 to F3 fibrosis coincides with a key pathophysiological transition point in disease natural history, with n = 73 metabolites altered.
Conclusions
Analysis of circulating metabolites provides important insights into the metabolic changes during NAFLD progression, revealing metabolic signatures across the NAFLD spectrum and features that are specific to NAFL, NASH, and fibrosis. The F2–F3 transition marks a critical metabolic transition point in NAFLD pathogenesis, with the data pointing to the pathophysiological importance of metabolic stress and specifically oxidative stress.
Lay summary
Non-alcoholic fatty liver disease is characterised by the build-up of fat in the liver, which progresses to liver dysfunction, scarring, and irreversible liver failure, and is markedly increasing in its prevalence worldwide. Here, we measured lipids and other small molecules (metabolites) in the blood with the aim of providing a comprehensive molecular overview of fat build-up, liver fibrosis, and diagnosed severity. We identify a key metabolic ‘watershed’ in the progression of liver damage, separating severe disease from mild, and show that specific lipid and metabolite profiles can help distinguish and/or define these cases.
Graphical abstract
Highlights
-
•
We assembled a highly phenotyped and characterised cohort of patients with NAFLD across the full spectrum of NAFLD.
-
•
Lipidomic and metabolomic interrogation of this dataset reveals crucial metabolic tipping point of NAFLD at fibrosis stage F2–F3.
-
•
NAFLD processes of fibrosis, steatosis, and NASH progression parallel distinct changes in metabolomic profiles.
-
•
Oxidative stress-buffering potential of the liver via ether lipids appears 1 of the key changes en route to late-stage NASH.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Design and validation of a histological scoring system for nonalcoholic fatty liver disease.
- Record: found
- Abstract: found
- Article: not found
Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention
- Record: found
- Abstract: found
- Article: not found