- Record: found
- Abstract: found
- Article: found
3M microfoam™ surgical tape prevents nasal pressure injury associated with nasotracheal intubation: A randomized double-blind trial

Read this article at
Background:
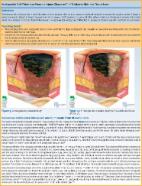
3M microfoam™ surgical tape (3ST: 3M Japan Limited) is used for pressure wound control of medical equipment. It is cushioned and can be fitted to any body part. Here we investigated whether 3ST prevents nasal pressure injury associated with nasotracheal intubation (NTI).
Methods:
We conducted a prospective, randomized double-blind study, enrolling 63 patients aged 20 to 70 years, who underwent general anesthesia with NTI. They were divided into 2 groups; those treated with 3ST (group S; n = 31) and control (group C; n = 31). After NTI and before securing the nasotracheal tube, a 35 × 25 mm 3ST was used to protect the nasal wing in group S, and the nasotracheal tube was fixed in place after NTI without protection in group C. The primary outcome was the presence or absence of nasal pressure injury after extubation. The Chi-Square test was used to assess the association between the 2 categorical variables.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: found
Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System
- Record: found
- Abstract: found
- Article: not found
Introduction to sample size determination and power analysis for clinical trials.
- Record: found
- Abstract: not found
- Article: not found