- Record: found
- Abstract: found
- Article: found
Efficacy of intrathecal methotrexate in children with high-risk medulloblastoma over three years: a retrospective study from a single center
Read this article at
Abstract
Purpose
Chemotherapy is commonly used for treatment in children over three years old with high-risk medulloblastoma(MB). However, little is currently known about the therapeutic benefits and side effects of intrathecal methotrexate(MTX), warranting further research.
Methods
In this retrospective study, patients who received intrathecal MTX during chemotherapy were included in the MTX group (n = 32), and patients that only underwent cerebrospinal fluid (CSF) cytology analysis were assigned to the control group (n = 14).
Results
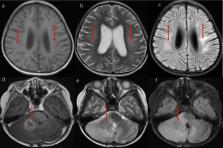
In the MTX group, 27(84.38%) patients had metastatic disease, 3(9.38%) had diffuse anaplasia, and 3(9.38%) had residual disease greater than 1.5 cm 2. Molecular subgroup classification was available for 28(87.5%) patients. In the control group, 8(57.14%) patients had metastatic disease, 3(27.27%) had diffuse anaplasia, and 6(42.86%) had residual disease greater than 1.5 cm 2. Molecular subgroup classification was available for 6(42.86%) patients. The 5-year progression-free survival was 70.99% and the 5-year overall survival was 72.99% for the MTX group, and the corresponding values were 41.67% and 50% for the control group, respectively. 6 (18.75%) patients in the MTX group with group 4 disease developed MTX-related acute leukoencephalopathy and one of them died.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
The 2007 WHO Classification of Tumours of the Central Nervous System
- Record: found
- Abstract: found
- Article: not found
Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial.
- Record: found
- Abstract: found
- Article: not found