- Record: found
- Abstract: found
- Article: found
Potential of Lactobacillus plantarum CCFM639 in Protecting against Aluminum Toxicity Mediated by Intestinal Barrier Function and Oxidative Stress
Read this article at
Abstract
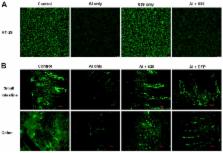
Aluminum (Al) is a ubiquitous metal that can seriously harm the health of animals and humans. In our previous study, we demonstrated that Lactobacillus plantarum CCFM639 can decrease Al burden in the tissues of mice by inhibiting intestinal Al absorption. The main aim of the present research was to investigate whether the protection by the strain is also associated with enhancement of the intestinal barrier, alleviation of oxidative stress and modulation of the inflammatory response. In an in vitro cell model, two protection modes (intervention and therapy) were examined and the results indicated that L. plantarum CCFM639 alleviated Al-induced cytotoxicity. In a mouse model, L. plantarum CCFM639 treatment was found to significantly alleviate oxidative stress in the intestinal tract, regulate the function of the intestinal mucosal immune system, restore the integrity of tight junction proteins and maintain intestinal permeability. These results suggest that in addition to Al sequestration, L. plantarum CCFM639 can also inhibit Al absorption by protecting the intestinal barrier, alleviating Al-induced oxidative stress and inflammatory response. Therefore, L. plantarum CCFM639 has the potential to be a dietary supplement ingredient that provides protection against Al-induced gut injury.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier.
- Record: found
- Abstract: found
- Article: not found
Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth.
- Record: found
- Abstract: found
- Article: not found