- Record: found
- Abstract: found
- Article: found
The Endothelial Transcription Factor ERG Promotes Vascular Stability and Growth through Wnt/β-Catenin Signaling

Read this article at
Summary
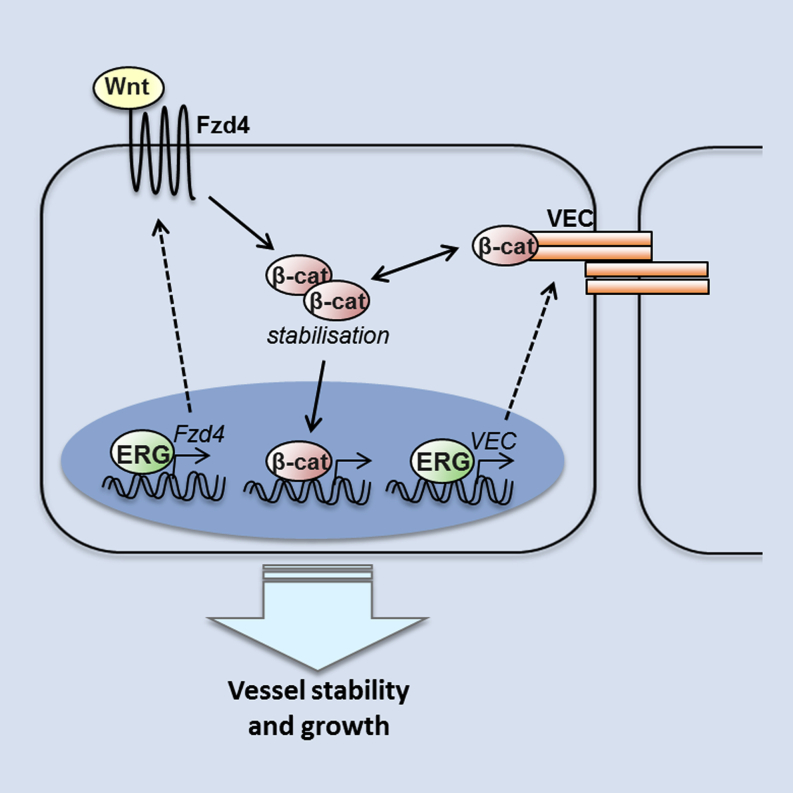
Blood vessel stability is essential for embryonic development; in the adult, many diseases are associated with loss of vascular integrity. The ETS transcription factor ERG drives expression of VE-cadherin and controls junctional integrity. We show that constitutive endothelial deletion of ERG ( Erg cEC-KO ) in mice causes embryonic lethality with vascular defects. Inducible endothelial deletion of ERG ( Erg iEC-KO ) results in defective physiological and pathological angiogenesis in the postnatal retina and tumors, with decreased vascular stability. ERG controls the Wnt/β-catenin pathway by promoting β-catenin stability, through signals mediated by VE-cadherin and the Wnt receptor Frizzled-4. Wnt signaling is decreased in ERG-deficient endothelial cells; activation of Wnt signaling with lithium chloride, which stabilizes β-catenin levels, corrects vascular defects in Erg cEC-KO embryos. Finally, overexpression of ERG in vivo reduces permeability and increases stability of VEGF-induced blood vessels. These data demonstrate that ERG is an essential regulator of angiogenesis and vascular stability through Wnt signaling.
Graphical Abstract
Highlights
Abstract
Birdsey, Shah et al. show that the endothelial ETS factor ERG controls Wnt/β-catenin signaling by promoting β-catenin stability, through pathways mediated by VE-cadherin and the Wnt receptor Frizzled-4. In vivo, ERG overexpression stabilizes VEGF-dependent angiogenesis. Thus, ERG is an essential regulator of angiogenesis and vascular stability through Wnt signaling.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Molecular regulation of vessel maturation.
- Record: found
- Abstract: found
- Article: not found
Wnt/β-Catenin/Tcf Signaling Induces the Transcription of Axin2, a Negative Regulator of the Signaling Pathway
- Record: found
- Abstract: found
- Article: not found