- Record: found
- Abstract: found
- Article: found
Why clinical trial outcomes fail to translate into benefits for patients
Read this article at
Abstract
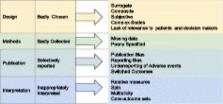
Clinical research should ultimately improve patient care. For this to be possible, trials must evaluate outcomes that genuinely reflect real-world settings and concerns. However, many trials continue to measure and report outcomes that fall short of this clear requirement. We highlight problems with trial outcomes that make evidence difficult or impossible to interpret and that undermine the translation of research into practice and policy. These complex issues include the use of surrogate, composite and subjective endpoints; a failure to take account of patients’ perspectives when designing research outcomes; publication and other outcome reporting biases, including the under-reporting of adverse events; the reporting of relative measures at the expense of more informative absolute outcomes; misleading reporting; multiplicity of outcomes; and a lack of core outcome sets. Trial outcomes can be developed with patients in mind, however, and can be reported completely, transparently and competently. Clinicians, patients, researchers and those who pay for health services are entitled to demand reliable evidence demonstrating whether interventions improve patient-relevant clinical outcomes.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: found
The prevention and handling of the missing data
- Record: found
- Abstract: found
- Article: not found
Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial.
- Record: found
- Abstract: found
- Article: not found