- Record: found
- Abstract: found
- Article: found
Costimulation Blockade in Kidney Transplant Recipients
Read this article at
Abstract
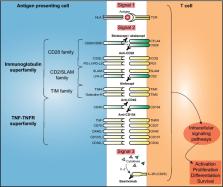
Costimulation between T cells and antigen-presenting cells is essential for the regulation of an effective alloimmune response and is not targeted with the conventional immunosuppressive therapy after kidney transplantation. Costimulation blockade therapy with biologicals allows precise targeting of the immune response but without non-immune adverse events. Multiple costimulation blockade approaches have been developed that inhibit the alloimmune response in kidney transplant recipients with varying degrees of success. Belatacept, an immunosuppressive drug that selectively targets the CD28-CD80/CD86 pathway, is the only costimulation blockade therapy that is currently approved for kidney transplant recipients. In the last decade, belatacept therapy has been shown to be a promising therapy in subgroups of kidney transplant recipients; however, the widespread use of belatacept has been tempered by an increased risk of acute kidney transplant rejection. The purpose of this review is to provide an overview of the costimulation blockade therapies that are currently in use or being developed for kidney transplant indications.
Related collections
Most cited references126
- Record: found
- Abstract: found
- Article: not found
CTLA-4 is a second receptor for the B cell activation antigen B7
- Record: found
- Abstract: found
- Article: not found