- Record: found
- Abstract: found
- Article: found
Effect of Food on the Pharmacokinetics of Ertugliflozin and Its Fixed‐Dose Combinations Ertugliflozin/Sitagliptin and Ertugliflozin/Metformin
Read this article at
Abstract
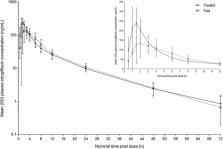
Ertugliflozin, an inhibitor of sodium‐glucose cotransporter 2, is approved in the United States and European Union for the treatment of type 2 diabetes in adults, both as monotherapy and as part of fixed‐dose combination (FDC) therapies with either sitagliptin or immediate‐release metformin. The effect of a standard, high‐fat breakfast on the pharmacokinetics of the highest strengths of ertugliflozin monotherapy (15 mg), ertugliflozin/sitagliptin FDC (15‐/100‐mg), and ertugliflozin/metformin FDC (7.5‐/1000‐mg) tablets was evaluated. In 3 separate open‐label, 2‐period, 2‐sequence, single‐dose, crossover studies, 14 healthy subjects per study were randomized to receive either ertugliflozin monotherapy or FDC tablets comprising ertugliflozin and sitagliptin or ertugliflozin and metformin under fasted and fed (or vice versa) conditions. Food did not meaningfully affect the pharmacokinetics of ertugliflozin, sitagliptin, or metformin. For FDCs, the effect of food was consistent with that described for individual components. All treatments were well tolerated. Ertugliflozin and ertugliflozin/sitagliptin FDC tablets can be administered without regard to meals. As metformin is administered with meals because of its gastrointestinal side effects, the ertugliflozin/metformin FDC should also be administered with meals.
Related collections
Most cited references15
- Record: found
- Abstract: found
- Article: not found
Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone.
- Record: found
- Abstract: found
- Article: not found
Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus.
- Record: found
- Abstract: found
- Article: not found