- Record: found
- Abstract: found
- Article: found
Prominent Levels of the Profibrotic Chemokine CCL18 during Peritonitis: In Vitro Downregulation by Vitamin D Receptor Agonists
Read this article at
Abstract
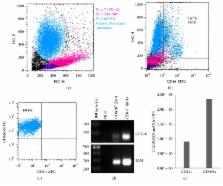
Peritoneal dialysis (PD) is used as a renal replacement therapy, which can be limited by peritoneal membrane ultrafiltration failure (UFF) secondary to fibrotic processes. Peritonitis, a frequent complication of PD, is a major risk factor for peritoneal membrane fibrosis and UFF. Low peritoneal levels of the chemokine CCL18 are associated with preservation of peritoneal membrane function in PD. Given that CCL18 is involved in fibrotic processes and recurrent peritonitis, it is a risk factor for peritoneal membrane failure; thus, we evaluated CCL18 concentrations in peritoneal effluents from patients undergoing peritonitis episodes. Pharmacological interventions aimed at diminishing the production of CCL18 were also explored. Fivefold higher CCL18 peritoneal concentrations were found during acute bacterial peritonitis, in parallel with the increased infiltration of macrophages. Unexpectedly, CCL18 was also highly (50-fold) increased during sterile eosinophilic peritonitis, and peritoneal eosinophils were found to express CCL18. In vitro treatment of peritoneal macrophages with the vitamin D receptor agonist paricalcitol was able to reduce the secretion and the expression of CCL18 in isolated peritoneal macrophages. In conclusion, our study suggests that the chemokine CCL18 can be a mediator of peritoneal membrane failure associated with peritonitis episodes as well as providing a new potential therapeutic target.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation.
- Record: found
- Abstract: found
- Article: not found
A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18.
- Record: found
- Abstract: found
- Article: not found