- Record: found
- Abstract: found
- Article: found
STAT3 is Overactivated in Gastric Cancer Stem-Like Cells
Read this article at
Abstract
Objective
Gastric cancer (GC) is widely associated with chronic inflammation. The pro inflammatory microenvironment provides conditions that disrupt stem/progenitor cell proliferation and differentiation. The signal transducer and activator of transcrip- tion-3 (STAT3) signaling pathway is involved in inflammation and also contributes to the maintenance of embryonic stem cell (ESCs) pluripotency. Here, we have investi- gated the activation status of STAT3 in GC stem-like cells (GCSLCs).
Materials and Methods
In this experimental research, CSLCs derived from the human GC cell line MKN-45 and patient specimens, through spheroid body formation, character- ized and then assayed for the STAT3 transcription factor expression in mRNA and protein level further to its activation.
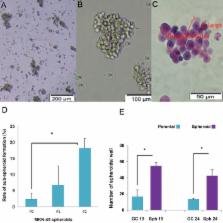
Results
Spheroid cells showed higher potential for spheroid formation than the pa- rental cells. Furthemore, stemness genes NANOG, c-MYC and SOX-2 were over expressed in spheroids of MKN-45 and in patient samples. In MKN-45 spheroid cells, epithelial mesenchymal transition (EMT) related markers CDH2, SNAIL2, TWIST and VIMENTIN were upregulated (P<0.05), but we observed no change in expression of the E-cadherin epithelial marker. These cells exhibited more resistance to docetaxel (DTX) when compared with parental cells (P<0.05) according to the MTS assay. Al- though immunostaining and Western blotting showed expression of the STAT3 pro- tein in both spheroids and parents, the mRNA level of STAT3 in spheroids was higher than the parents. Nuclear translocation of STAT3 was accompanied by more intensive phospho-STAT3 (p-STAT3) in spheroid structures relative to the parent cells accord- ing to flow cytometry analysis (P<0.05).
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
The JAK2/STAT3 signaling pathway is required for growth of CD44⁺CD24⁻ stem cell-like breast cancer cells in human tumors.
- Record: found
- Abstract: not found
- Article: not found
Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994.
- Record: found
- Abstract: found
- Article: not found