- Record: found
- Abstract: found
- Article: found
Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders
Read this article at
Abstract
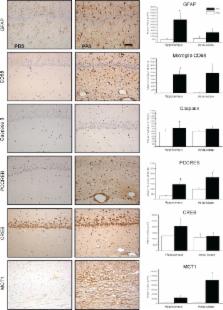
Recent evidence suggests potential, but unproven, links between dietary, metabolic, infective, and gastrointestinal factors and the behavioral exacerbations and remissions of autism spectrum disorders (ASDs). Propionic acid (PPA) and its related short-chain fatty acids (SCFAs) are fermentation products of ASD-associated bacteria ( Clostridia, Bacteriodetes, Desulfovibrio). SCFAs represent a group of compounds derived from the host microbiome that are plausibly linked to ASDs and can induce widespread effects on gut, brain, and behavior. Intraventricular administration of PPA and SCFAs in rats induces abnormal motor movements, repetitive interests, electrographic changes, cognitive deficits, perseveration, and impaired social interactions. The brain tissue of PPA-treated rats shows a number of ASD-linked neurochemical changes, including innate neuroinflammation, increased oxidative stress, glutathione depletion, and altered phospholipid/acylcarnitine profiles. These directly or indirectly contribute to acquired mitochondrial dysfunction via impairment in carnitine-dependent pathways, consistent with findings in patients with ASDs. Of note, common antibiotics may impair carnitine-dependent processes by altering gut flora favoring PPA-producing bacteria and by directly inhibiting carnitine transport across the gut. Human populations that are partial metabolizers of PPA are more common than previously thought. PPA has further bioactive effects on neurotransmitter systems, intracellular acidification/calcium release, fatty acid metabolism, gap junction gating, immune function, and alteration of gene expression that warrant further exploration. These findings are consistent with the symptoms and proposed underlying mechanisms of ASDs and support the use of PPA infusions in rats as a valid animal model of the condition. Collectively, this offers further support that gut-derived factors, such as dietary or enteric bacterially produced SCFAs, may be plausible environmental agents that can trigger ASDs or ASD-related behaviors and deserve further exploration in basic science, agriculture, and clinical medicine.
Related collections
Most cited references198
- Record: found
- Abstract: found
- Article: not found
Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation.
- Record: found
- Abstract: found
- Article: not found
Pyrosequencing study of fecal microflora of autistic and control children.
- Record: found
- Abstract: found
- Article: not found