- Record: found
- Abstract: found
- Article: found
Roles of Calcium Regulating MicroRNAs in Cardiac Ischemia-Reperfusion Injury
Read this article at
Abstract
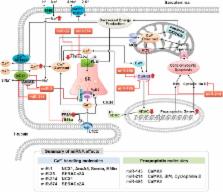
Cardiac Ca 2+ cycling and signaling are closely associated with cardiac function. Changes in cellular Ca 2+ homeostasis may lead to aberrant cardiac rhythm and may play a critical role in the pathogenesis of cardiac diseases, due to their exacerbation of heart failure. MicroRNAs (miRNAs) play a key role in the regulation of gene expression at the post-transcriptional level and participate in regulating diverse biological processes. The emerging evidence indicates that the expression profiles of miRNAs vary among human diseases, including cardiovascular diseases. Cardiac Ca 2+-handling and signaling proteins are also regulated by miRNAs. Given the relationship between cardiac Ca 2+ homeostasis and signaling and miRNA, Ca 2+-related miRNAs may serve as therapeutic targets during the treatment of heart failure. In this review, we summarize the knowledge currently available regarding the role of Ca 2+ in cardiac function, as well as changes in Ca 2+ cycling and homeostasis and the handling of these processes by miRNAs during cardiac ischemia-reperfusion injury.
Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: not found
Circulating microRNAs: Association with disease and potential use as biomarkers.
- Record: found
- Abstract: found
- Article: not found
Inhibition of miR-15 protects against cardiac ischemic injury.

- Record: found
- Abstract: found
- Article: found