- Record: found
- Abstract: found
- Article: found
The Role of Rac GTPase in Dendritic Spine Morphogenesis and Memory
Read this article at
Abstract
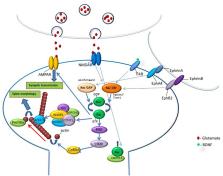
The ability to form memories in the brain is needed for daily functions, and its impairment is associated with human mental disorders. Evidence indicates that long-term memory (LTM)-related processes such as its consolidation, extinction and forgetting involve changes of synaptic efficacy produced by alterations in neural transmission and morphology. Modulation of the morphology and number of dendritic spines has been proposed to contribute to changes in neuronal transmission mediating such LTM-related processes. Rac GTPase activity is regulated by synaptic activation and it can affect spine morphology by controlling actin-regulatory proteins. Recent evidence shows that changes in Rac GTPase activity affect memory consolidation, extinction, erasure and forgetting and can affect spine morphology in brain areas that mediate these behaviors. Altered Rac GTPase activity is associated with abnormal spine morphology and brain disorders. By affecting Rac GTPase activity we can further understand the roles of spine morphogenesis in memory. Moreover, manipulation of Rac GTPase activity may serve as a therapeutic tool for the treatment of memory-related brain diseases.
Related collections
Most cited references105
- Record: found
- Abstract: found
- Article: not found
Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization.
- Record: found
- Abstract: found
- Article: not found
Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics.
- Record: found
- Abstract: found
- Article: not found