- Record: found
- Abstract: found
- Article: found
Using human induced pluripotent stem cells to model cerebellar disease: Hope and hype
Abstract
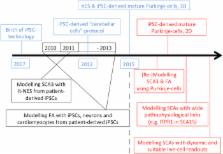
The cerebellum forms a highly ordered and indispensible component of motor function within the adult neuraxis, consisting of several distinct cellular subtypes. Cerebellar disease, through a variety of genetic and acquired causes, results in the loss of function of defined subclasses of neurons, and remains a significant and untreatable health care burden. The scarcity of therapies in this arena can partially be explained by unresolved disease mechanisms due to inaccessibility of human cerebellar neurons in a relevant experimental context where initiating disease mechanisms could be functionally elucidated, or drug screens conducted. In this review we discuss the potential promise of human induced pluripotent stem cells (hiPSCs) for regenerative neurology, with a particular emphasis on in vitro modelling of cerebellar degeneration. We discuss progress made thus far using hiPSC-based models of neurodegeneration, noting the relatively slower pace of discovery made in modelling cerebellar dysfunction. We conclude by speculating how strategies attempting cerebellar differentiation from hiPSCs can be refined to allow the generation of accurate disease models. This in turn will permit a greater understanding of cerebellar pathophysiology to inform mechanistically rationalised therapies, which are desperately needed in this field.
Related collections
Most cited references104
- Record: found
- Abstract: found
- Article: not found
Induction of pluripotent stem cells from adult human fibroblasts by defined factors.
- Record: found
- Abstract: found
- Article: not found
Cerebral organoids model human brain development and microcephaly
- Record: found
- Abstract: found
- Article: not found