- Record: found
- Abstract: found
- Article: found
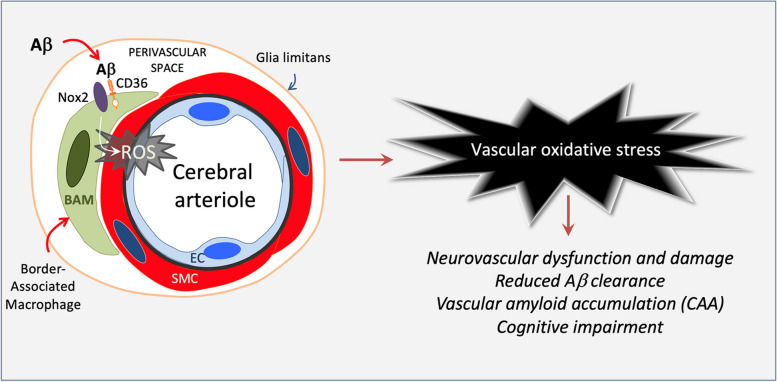
Border-associated macrophages promote cerebral amyloid angiopathy and cognitive impairment through vascular oxidative stress

Read this article at
Abstract
Background
Cerebral amyloid angiopathy (CAA) is a devastating condition common in patients with Alzheimer’s disease but also observed in the general population. Vascular oxidative stress and neurovascular dysfunction have been implicated in CAA but the cellular source of reactive oxygen species (ROS) and related signaling mechanisms remain unclear. We tested the hypothesis that brain border-associated macrophages (BAM), yolk sac-derived myeloid cells closely apposed to parenchymal and leptomeningeal blood vessels, are the source of radicals through the Aβ-binding innate immunity receptor CD36, leading to neurovascular dysfunction, CAA, and cognitive impairment.
Methods
Tg2576 mice and WT littermates were transplanted with CD36 −/− or CD36 +/+ bone marrow at 12-month of age and tested at 15 months. This approach enables the repopulation of perivascular and leptomeningeal compartments with CD36 −/− BAM. Neurovascular function was tested in anesthetized mice equipped with a cranial window in which cerebral blood flow was monitored by laser-Doppler flowmetry. Amyloid pathology and cognitive function were also examined.
Results
The increase in blood flow evoked by whisker stimulation (functional hyperemia) or by endothelial and smooth muscle vasoactivity was markedly attenuated in WT → Tg2576 chimeras but was fully restored in CD36 −/− → Tg2576 chimeras, in which BAM ROS production was suppressed. CAA-associated Aβ 1-40, but not Aβ 1-42, was reduced in CD36 −/− → Tg2576 chimeras. Similarly, CAA, but not parenchymal plaques, was reduced in CD36 −/− → Tg2576 chimeras. These beneficial vascular effects were associated with cognitive improvement. Finally, CD36 −/− mice were able to more efficiently clear exogenous Aβ 1-40 injected into the neocortex or the striatum.
Conclusions
CD36 deletion in BAM suppresses ROS production and rescues the neurovascular dysfunction and damage induced by Aβ. CD36 deletion in BAM also reduced brain Aβ 1-40 and ameliorated CAA without affecting parenchyma plaques. Lack of CD36 enhanced the vascular clearance of exogenous Aβ. Restoration of neurovascular function and attenuation of CAA resulted in a near complete rescue of cognitive function. Collectively, these data implicate brain BAM in the pathogenesis of CAA and raise the possibility that targeting BAM CD36 is beneficial in CAA and other conditions associated with vascular Aβ deposition and damage.
Related collections
Most cited references100
- Record: found
- Abstract: found
- Article: not found
The antibody aducanumab reduces Aβ plaques in Alzheimer's disease.
- Record: found
- Abstract: found
- Article: not found
Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice.
- Record: found
- Abstract: found
- Article: not found