- Record: found
- Abstract: found
- Article: found
MET inhibitor tepotinib antagonizes multidrug resistance mediated by ABCG2 transporter: In vitro and in vivo study

Read this article at
Abstract
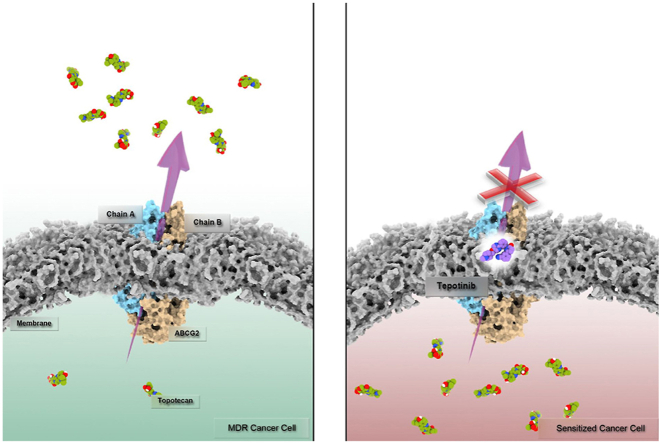
Overexpression of ABCG2 transporter in cancer cells has been linked to the development of multidrug resistance (MDR), an obstacle to cancer therapy. Our recent study uncovered that the MET inhibitor, tepotinib, is a potent reversal agent for ABCB1-mediated MDR. In the present study, we reported for the first time that the MET inhibitor tepotinib can also reverse ABCG2-mediated MDR in vitro and in vivo by directly binding to the drug-binding site of ABCG2 and reversibly inhibiting ABCG2 drug efflux activity, therefore enhancing the cytotoxicity of substrate drugs in drug-resistant cancer cells. Furthermore, the ABCB1/ABCG2 double-transfected cell model and ABCG2 gene knockout cell model demonstrated that tepotinib specifically inhibits the two MDR transporters. In mice bearing drug-resistant tumors, tepotinib increased the intratumoral accumulation of ABCG2 substrate drug topotecan and enhanced its antitumor effect. Therefore, our study provides a new potential of repositioning tepotinib as an ABCG2 inhibitor and combining tepotinib with substrate drugs to antagonize ABCG2-mediated MDR.
Graphical abstract
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading.
- Record: found
- Abstract: found
- Article: not found
Revisiting the role of ABC transporters in multidrug-resistant cancer
- Record: found
- Abstract: found
- Article: not found