- Record: found
- Abstract: found
- Article: found
Clinical and Experimental Pancreatic Islet Transplantation to Striated Muscle : Establishment of a Vascular System Similar to That in Native Islets
Read this article at
Abstract
OBJECTIVE
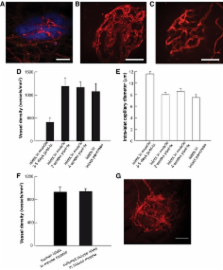
Curing type 1 diabetes by transplanting pancreatic islets into the liver is associated with poor long-term outcome and graft failure at least partly due to inadequate graft revascularization. The aim of the current study was to evaluate striated muscle as a potential angiogenic site for islet transplantation.
RESEARCH DESIGN AND METHODS
The current study presents a new experimental model that is found to be applicable to clinical islet transplantation. Islets were implanted into striated muscle and intraislet vascular density and blood flow were visualized with intravital and confocal microscopy in mice and by magnetic resonance imaging in three autotransplanted pancreatectomized patients. Mice were rendered neutropenic by repeated injections of Gr-1 antibody, and diabetes was induced by alloxan treatment.
RESULTS
Contrary to liver-engrafted islets, islets transplanted to mouse muscle were revascularized with vessel densities and blood flow entirely comparable with those of islets within intact pancreas. Initiation of islet revascularization at the muscular site was dependent on neutrophils, and the function of islets transplanted to muscle was proven by curing diabetic mice. The experimental data were confirmed in autotransplanted patients where higher plasma volumes were measured in islets engrafted in forearm muscle compared with adjacent muscle tissue through high-resolution magnetic resonance imaging.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Five-year follow-up after clinical islet transplantation.
- Record: found
- Abstract: found
- Article: not found
Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis.
- Record: found
- Abstract: found
- Article: not found