- Record: found
- Abstract: found
- Article: found
Endothelial-to-mesenchymal transition in anticancer therapy and normal tissue damage
Read this article at
Abstract
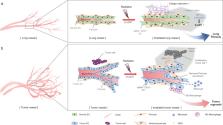
Endothelial-to-mesenchymal transition (EndMT) involves the phenotypic conversion of endothelial-to-mesenchymal cells, and was first discovered in association with embryonic heart development. EndMT can regulate various processes, such as tissue fibrosis and cancer. Recent findings have shown that EndMT is related to resistance to cancer therapy, such as chemotherapy, antiangiogenic therapy, and radiation therapy. Based on the known effects of EndMT on the cardiac toxicity of anticancer therapy and tissue damage of radiation therapy, we propose that EndMT can be targeted as a strategy for overcoming tumor resistance while reducing complications, such as tissue damage. In this review, we discuss EndMT and its roles in damaging cardiac and lung tissues, as well as EndMT-related effects on tumor vasculature and resistance in anticancer therapy. Modulating EndMT in radioresistant tumors and radiation-induced tissue fibrosis can especially increase the efficacy of radiation therapy. In addition, we review the role of hypoxia and reactive oxygen species as the main stimulating factors of tissue damage due to vascular damage and EndMT. We consider drugs that may be clinically useful for regulating EndMT in various diseases. Finally, we argue the importance of EndMT as a therapeutic target in anticancer therapy for reducing tissue damage.
Cell biology: effect of changes in cell type on disease
A process of cellular conversion known as endothelial-to-mesenchymal transition (EndMT) may offer a valuable target for treating cancer and other diseases. In EndMT, the cells lining blood vessels undergo a striking change in shape and physiology, acquiring features of cells called fibroblasts. Fibroblasts form the body’s connective tissue, but also produce scar tissue that impairs organ function. Researchers led by Yoon-Jin Lee of the Korea Institute of Radiological & Medical Sciences in Seoul, South Korea, have reviewed the impact of this transformation on human disease. EndMT is seen as a prelude to heart failure, in lung tissue affected by pulmonary fibrosis, and within tumors, where the process recruits cells that further stimulate cancer progression. The authors highlight the potential of using drugs that target EndMT to bolster the efficacy and safety of tumor therapy.
Related collections
Most cited references90
- Record: found
- Abstract: found
- Article: not found
Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases.
- Record: found
- Abstract: not found
- Article: not found
Cardiovascular Toxic Effects of Targeted Cancer Therapies.
- Record: found
- Abstract: found
- Article: not found