- Record: found
- Abstract: found
- Article: not found
Onset of Propene Oligomerization Reactivity in ZSM-5 Studied by Inelastic Neutron Scattering Spectroscopy

Read this article at
Abstract
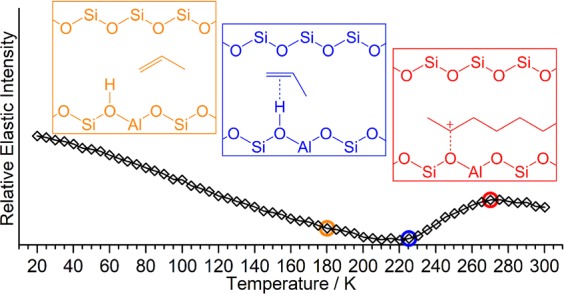
The techniques of quasi-elastic and inelastic neutron scattering (QENS and INS) are applied to investigate the oligomerization of propene over a ZSM-5 zeolite. Investigations are performed at low temperatures, allowing identification of the onset of the oligomerization reaction and observation of the low-energy spectral changes due to intermediate formation that are difficult to observe by optical methods. Oligomerization proceeds via formation of a hydrogen-bonded precursor by an interaction of the propene with an internal acid site followed by protonation and chain growth with protonation being the rate-limiting step. The use of quasi-elastic neutron scattering to observe changes in system mobility with temperature via the elastic window scan technique allows identification of the active temperature range where catalyst activity commences and permits targeting of the more time-consuming INS investigations to conditions of interest. From examination of the product’s spectrum, the structure of the resulting oligomer is deduced to be primarily linear.
Related collections
Most cited references30
- Record: found
- Abstract: not found
- Article: not found
Mantid—Data analysis and visualization package for neutron scattering and\(\mathrm{\mu }\)SR experiments
- Record: found
- Abstract: not found
- Article: not found
Recent trends and fundamental insights in the methanol-to-hydrocarbons process
- Record: found
- Abstract: found
- Article: not found
