- Record: found
- Abstract: found
- Article: found
Interactions between plant endomembrane systems and the actin cytoskeleton
Read this article at
Abstract
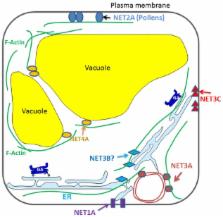
Membrane trafficking, organelle movement, and morphogenesis in plant cells are mainly controlled by the actin cytoskeleton. Not all proteins that regulate the cytoskeleton and membrane dynamics in animal systems have functional homologs in plants, especially for those proteins that form the bridge between the cytoskeleton and membrane; the membrane-actin adaptors. Their nature and function is only just beginning to be elucidated and this field has been greatly enhanced by the recent identification of the NETWORKED (NET) proteins, which act as membrane-actin adaptors. In this review, we will summarize the role of the actin cytoskeleton and its regulatory proteins in their interaction with endomembrane compartments and where they potentially act as platforms for cell signaling and the coordination of other subcellular events.
Related collections
Most cited references94
- Record: found
- Abstract: found
- Article: not found
Arabidopsis LTPG is a glycosylphosphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface.
- Record: found
- Abstract: found
- Article: not found
The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function.
- Record: found
- Abstract: found
- Article: not found