- Record: found
- Abstract: found
- Article: found
Compound K ameliorates airway inflammation and mucus secretion through the regulation of PKC signaling in vitro and in vivo
Read this article at
Abstract
Background
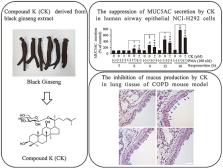
Cigarette smoke (CS) is considered a principal cause of chronic obstructive pulmonary disease (COPD) and is associated with mucus hypersecretion and airway inflammation. Ginsenoside compound K (CK), a product of ginsenoside metabolism, has various biological activities. Studies on the effects of CK for the treatment of COPD and mucus hypersecretion, including the underlying signaling mechanism, have not yet been conducted.
Methods
To study the protective effects and molecular mechanism of CK, phorbol 12-myristate 13-acetate (PMA)-induced human airway epithelial (NCI–H292) cells were used as a cellular model of airway inflammation. An experimental mouse COPD model was also established via CS inhalation and intranasal administration of lipopolysaccharide. Mucin 5AC (MUC5AC), monocyte chemoattractant protein-1, tumor necrosis factor-α (TNF-α), and interleukin-6 secretion, as well as elastase activity and reactive oxygen species production, were determined through enzyme-linked immunosorbent assay. Inflammatory cell influx and mucus secretion in mouse lung tissues were estimated using hematoxylin and eosin and periodic acid–schiff staining, respectively. PKCδ and its downstream signaling molecules were analyzed via western blotting.
Results
CK prevented the secretion of MUC5AC and TNF-α in PMA-stimulated NCI–H292 cells and exhibited a protective effect in COPD mice via the suppression of inflammatory mediators and mucus secretion. These effects were accompanied by an inactivation of PKCδ and related signaling in vitro and in vivo.
Graphical abstract
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Inflammatory mechanisms in patients with chronic obstructive pulmonary disease.
- Record: found
- Abstract: found
- Article: not found

