- Record: found
- Abstract: found
- Article: found
Upregulated LINE-1 Activity in the Fanconi Anemia Cancer Susceptibility Syndrome Leads to Spontaneous Pro-inflammatory Cytokine Production
Read this article at
Abstract
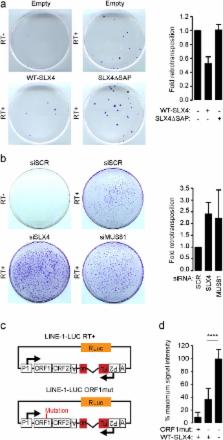
Fanconi Anemia (FA) is a genetic disorder characterized by elevated cancer susceptibility and pro-inflammatory cytokine production. Using SLX4 FANCP deficiency as a working model, we questioned the trigger for chronic inflammation in FA. We found that absence of SLX4 caused cytoplasmic DNA accumulation, including sequences deriving from active Long INterspersed Element-1 (LINE-1), triggering the cGAS-STING pathway to elicit interferon (IFN) expression. In agreement, absence of SLX4 leads to upregulated LINE-1 retrotransposition. Importantly, similar results were obtained with the FANCD2 upstream activator of SLX4. Furthermore, treatment of FA cells with the Tenofovir reverse transcriptase inhibitor (RTi), that prevents endogenous retrotransposition, decreased both accumulation of cytoplasmic DNA and pro-inflammatory signaling. Collectively, our data suggest a contribution of endogenous RT activities to the generation of immunogenic cytoplasmic nucleic acids responsible for inflammation in FA. The additional observation that RTi decreased pro-inflammatory cytokine production induced by DNA replication stress-inducing drugs further demonstrates the contribution of endogenous RTs to sustaining chronic inflammation. Altogether, our data open perspectives in the prevention of adverse effects of chronic inflammation in tumorigenesis.
Highlights
-
•
Cytoplasmic DNA, comprising LINE-1-derived sequences, elicits IFN expression via the cGAS-STING pathway in SLX4-deficiency.
-
•
Members of the Fanconi Anemia DNA repair pathway negatively regulate LINE-1 retrotransposition.
-
•
Endogenous reverse transcriptase activities contribute to spontaneous and chemotherapy-induced inflammation.
Chronic inflammation favors tumorigenesis, negatively influencing patient prognosis. Yet, the underlying molecular mechanisms are poorly understood. Here, we show that increased endogenous retroelement-associated reverse transcriptase activity contributes to generate immunogenic cytoplasmic nucleic acids susceptible of triggering a pro-inflammatory response in the Fanconi Anemia (FA) cancer susceptibility syndrome. In addition, treatment of FA cells or of cells exposed to replication stress inducing drugs, with a reverse transcriptase inhibitor, decreases pro-inflammatory signals. Altogether our data suggest the involvement of endogenous reverse transcriptase activities in sustaining pervasive chronic inflammation, opening therapeutic perspectives for preventing its impact on tumorigenesis.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Persistent DNA damage signaling triggers senescence-associated inflammatory cytokine secretion
- Record: found
- Abstract: found
- Article: not found
STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors.
- Record: found
- Abstract: found
- Article: not found