- Record: found
- Abstract: found
- Article: found
Cryo-EM structure of Mcm2-7 double hexamer on DNA suggests a lagging-strand DNA extrusion model
Read this article at
Significance
During initiation of DNA replication in eukaryotes, the origin recognition complex, with Cdc6 and Cdt1, assembles an inactive Mcm2-7 double hexamer on the dsDNA. Later, the double hexamer recruits Cdc45 and GINS to form two active and separate DNA helicases. The active Cdc45–Mcm2-7–GINS helicase encircles the leading strand while excluding the lagging strand. One of the fundamental unanswered questions is how each Mcm2-7 hexamer converts from binding dsDNA to binding one of the single strands. The structure of the double hexamer on dsDNA reveals how DNA interacts with key elements inside the central channel, leading us to propose a lagging-strand extrusion mechanism. This work advances our understanding of eukaryotic replication initiation.
Abstract
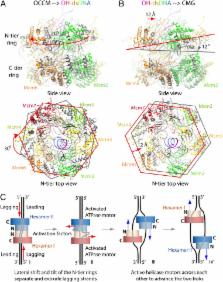
During replication initiation, the core component of the helicase—the Mcm2-7 hexamer—is loaded on origin DNA as a double hexamer (DH). The two ring-shaped hexamers are staggered, leading to a kinked axial channel. How the origin DNA interacts with the axial channel is not understood, but the interaction could provide key insights into Mcm2-7 function and regulation. Here, we report the cryo-EM structure of the Mcm2-7 DH on dsDNA and show that the DNA is zigzagged inside the central channel. Several of the Mcm subunit DNA-binding loops, such as the oligosaccharide–oligonucleotide loops, helix 2 insertion loops, and presensor 1 (PS1) loops, are well defined, and many of them interact extensively with the DNA. The PS1 loops of Mcm 3, 4, 6, and 7, but not 2 and 5, engage the lagging strand with an approximate step size of one base per subunit. Staggered coupling of the two opposing hexamers positions the DNA right in front of the two Mcm2–Mcm5 gates, with each strand being pressed against one gate. The architecture suggests that lagging-strand extrusion initiates in the middle of the DH that is composed of the zinc finger domains of both hexamers. To convert the Mcm2-7 DH structure into the Mcm2-7 hexamer structure found in the active helicase, the N-tier ring of the Mcm2-7 hexamer in the DH-dsDNA needs to tilt and shift laterally. We suggest that these N-tier ring movements cause the DNA strand separation and lagging-strand extrusion.
Related collections
Most cited references86
- Record: found
- Abstract: found
- Article: not found
DNA replication in eukaryotic cells.
- Record: found
- Abstract: found
- Article: not found
Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing.
- Record: found
- Abstract: found
- Article: not found
