- Record: found
- Abstract: found
- Article: found
L-Proline Induces a Mesenchymal-like Invasive Program in Embryonic Stem Cells by Remodeling H3K9 and H3K36 Methylation

Read this article at
Summary
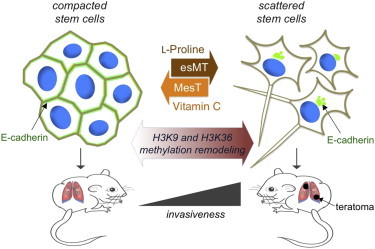
Metabolites are emerging as key mediators of crosstalk between metabolic flux, cellular signaling, and epigenetic regulation of cell fate. We found that the nonessential amino acid L-proline (L-Pro) acts as a signaling molecule that promotes the conversion of embryonic stem cells into mesenchymal-like, spindle-shaped, highly motile, invasive pluripotent stem cells. This embryonic-stem-cell-to-mesenchymal-like transition (esMT) is accompanied by a genome-wide remodeling of the H3K9 and H3K36 methylation status. Consistently, L-Pro-induced esMT is fully reversible either after L-Pro withdrawal or by addition of ascorbic acid (vitamin C), which in turn reduces H3K9 and H3K36 methylation, promoting a mesenchymal-like-to-embryonic-stem-cell transition (MesT). These findings suggest that L-Pro, which is produced by proteolytic remodeling of the extracellular matrix, may act as a microenvironmental cue to control stem cell behavior.
Graphical Abstract
Highlights
-
•
L-Pro induces a reversible embryonic-stem-to-mesenchymal-like transition (esMT)
-
•
The esMT is characterized by a dynamic redistribution of E-cadherin
-
•
L-Pro acts as an epigenetic modifier remodeling H3K9 and H3K36 methylation
-
•
L-Pro and vitamin C regulate esMT-MesT plasticity modulating H3K9/H3K36 methylation
Abstract
Comes et al. report that L-proline acts as an epigenetic modifier in embryonic stem cells (ESCs) inducing a reversible embryonic-stem-to-mesenchymal-like transition, which converts compacted ESCs into highly motile, invasive stem cells. Vitamin C counteracts L-proline promoting a mesenchymal-like-to-embryonic-stem-cell transition. These findings strengthen the role of the amino acids as key regulators of stem cell behavior.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Epithelial-mesenchymal transitions in development and disease.
- Record: found
- Abstract: found
- Article: not found