- Record: found
- Abstract: found
- Article: found
Intravenous levosimendan vs. dobutamine in acute decompensated heart failure patients on beta-blockers
Read this article at
Abstract
Aims
The aim of this study is to compare the effects of a 24 h intravenous infusion of levosimendan and a 48 h infusion of dobutamine on invasive haemodynamics in patients with acutely decompensated chronic NYHA class III–IV heart failure. All patients were receiving optimal oral therapy including a β-blocker.
Methods and results
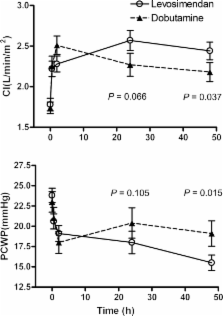
This was a multinational, randomized, double-blind, phase IV study in 60 patients; follow-up was 1 month. There was a significant increase in cardiac index and a significant decrease in pulmonary capillary wedge pressure (PCWP) at 24 and 48 h for both dobutamine and levosimendan. The improvement in cardiac index with levosimendan was not significantly different from dobutamine at 24 h ( P = 0.07), but became significant at 48 h (0.44 ± 0.56 vs. 0.66 ± 0.63 L/min/m 2; P = 0.04). At 24 h, the reduction in the mean change in PCWP from baseline was similar for levosimendan and dobutamine, however, at 48 h the difference was more marked for levosimendan (−3.6 ± 7.6 vs. −8.3 ± 6.7 mmHg; P = 0.02). No difference was observed between the groups for change in NYHA class, β-blocker use, hospitalizations, treatment discontinuations or rescue medication use. Reduction in B-type natriuretic peptide (BNP) was significantly greater with levosimendan at 48 h ( P = 0.03). According to physician's assessment, the improvement in fatigue ( P = 0.01) and dyspnoea ( P = 0.04) was in favour of dobutamine treatment, and hypotension was significantly more frequent with levosimendan ( P = 0.007). No increase in atrial fibrillation or ventricular tachycardia was seen in either group.
Related collections
Most cited references15
- Record: found
- Abstract: not found
- Article: not found
HUMAN EXPERIMENTATION. CODE OF ETHICS OF THE WORLD MEDICAL ASSOCIATION. DECLARATION OF HELSINKI.
- Record: found
- Abstract: found
- Article: not found
Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial.
- Record: found
- Abstract: found
- Article: not found