- Record: found
- Abstract: found
- Article: found
BDNF Outperforms TrkB Agonist 7,8,3′-THF in Preserving the Auditory Nerve in Deafened Guinea Pigs
Read this article at
Abstract
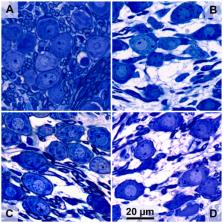
In deaf subjects using a cochlear implant (CI) for hearing restoration, the auditory nerve is subject to degeneration, which may negatively impact CI effectiveness. This nerve degeneration can be reduced by neurotrophic treatment. Here, we compare the preservative effects of the naturally occurring tyrosine receptor kinase B (TrkB) agonist brain-derived neurotrophic factor (BDNF) and the small-molecule TrkB agonist 7,8,3′-trihydroxyflavone (THF) on the auditory nerve in deafened guinea pigs. THF may be more effective than BDNF throughout the cochlea because of better pharmacokinetic properties. The neurotrophic compounds were delivered by placement of a gelatin sponge on the perforated round window membrane. To complement the histology of spiral ganglion cells (SGCs), electrically evoked compound action potential (eCAP) recordings were performed four weeks after treatment initiation. We analyzed the eCAP inter-phase gap (IPG) effect and measures derived from pulse-train evoked eCAPs, both indicative of SGC healthiness. BDNF but not THF yielded a significantly higher survival of SGCs in the basal cochlear turn than untreated controls. Regarding IPG effect and pulse-train responses, the BDNF-treated animals exhibited more normal responses than both untreated and THF-treated animals. We have thus confirmed the protective effect of BDNF, but we have not confirmed previously reported protective effects of THF with our clinically applicable delivery method.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases.
- Record: found
- Abstract: found
- Article: not found
A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone.
- Record: found
- Abstract: found
- Article: not found