- Record: found
- Abstract: found
- Article: not found
Evaluation of an immunochemotherapeutic protocol constituted of N-methyl meglumine antimoniate (Glucantime ®) and the recombinant Leish-110f ® + MPL-SE ® vaccine to treat canine visceral leishmaniasis
Summary
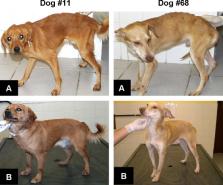
The evaluation of the efficacy of an immunochemotherapy protocol to treat symptomatic dogs naturally infected with Leishmania chagasi was studied. This clinical trial had the purpose to test the combination of N-methyl meglumine antimoniate (Glucantime ®) and the second generation recombinant vaccine Leish-110f ® plus the adjuvant MPL-SE ® to treat the canine leishmaniasis (CanL). Thirty symptomatic naturally infected mongrel dogs were divided into five groups. Animals received standard treatment with Glucantime ® or treatment with Glucantime ®/Leish-110f ® + MPL-SE ® as immunochemotherapy protocol. Additional groups received Leish-110f ® + MPL-SE ® only, MPL-SE ® only, or placebo. Evaluation of haematological, biochemical (renal and hepatic function) and plasmatic proteins, immunological (humoral and cellular immune response) and the parasitological test revealed improvement of the clinical parameters and parasitological cure in dogs in both chemotherapy alone and immunochemotherapy cohorts. However, the immunotherapy and immunochemotherapy cohorts had reduced number of deaths, higher survival probability, and specific cellular reactivity to leishmanial antigens, in comparison with chemotherapy cohort only and control groups (adjuvant alone and placebo).
These results support the notion of using well-characterized recombinant vaccine as an adjunct to improve the current chemotherapy of CanL.
Related collections
Most cited references52
- Record: found
- Abstract: not found
- Article: not found
Leishmaniasis--current chemotherapy and recent advances in the search for novel drugs.
- Record: found
- Abstract: found
- Article: not found
A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum.
- Record: found
- Abstract: found
- Article: not found
